Product Designer
Complaince Engineers, Product Owner, Software Engineer
Instructions for use (IFU) are required by the FDA for medical devices, and thus required for some of the products that Glidewell produces.
IFUs are printed on paper and mailed out with the product. Most likely, they are being thrown away as soon as the recipient receives the product. The process of creating, printing, and packaging the IFUs is cumbersome and expensive and is estimated to cost the company $273,000 a year.
In an effort to reduce costs and labor, a proposal came to the engineering and design group to find a way to offer them digitally to our customers. The FDA had no strict guidelines on digital IFUs at the time, so we looked to the EU guidelines for reference.
Because the compliance and regulatory affairs group also agreed that this was almost a "userless" project (they didn't think anyone actually read the IFUs or kept them with the product), the majority of the requirements for the project were to adhere to the EU guidelines.
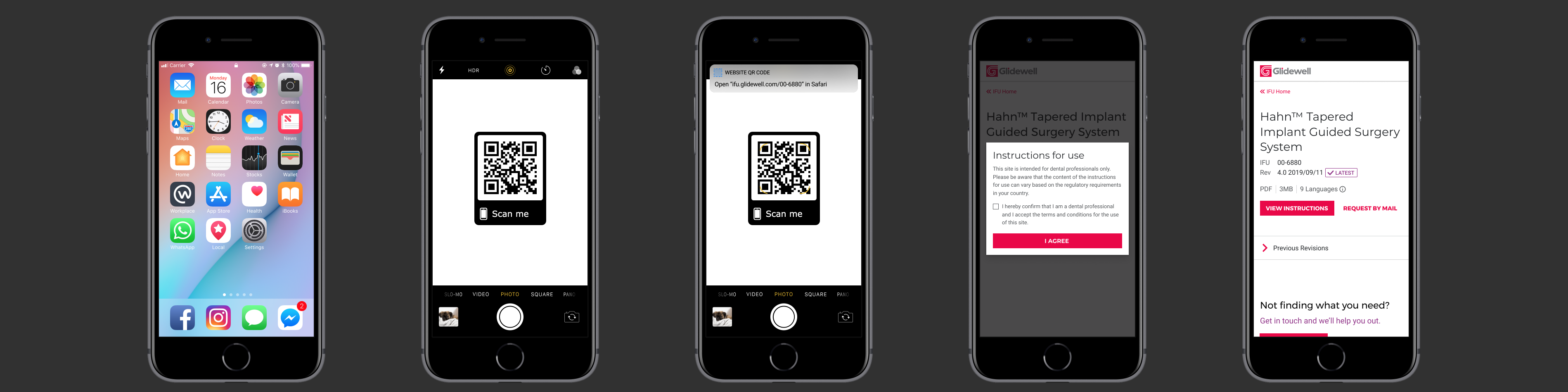
The key piece provided by regulatory affairs was to include a scannable QR code on the product label. The thought was that if any of the customers had questions about the product, they could scan it with their phone and find the IFU.
